Realizing the benefits of the adaptibility of Agile Development and the discipline of Quality Management Systems, Agile Quality Systems provides training and coaching to help medical device development organizations improve their performance, compliance, and team health.
For those new to Agile methods or those who need a refresher, Agile Quality Systems provides foundational training on Agile, beginning with the principles of the Agile Manifesto, then the foundational elements of common Agile frameworks including Scrum, Extreme Programming, Kanban, and Scaling Frameworks.
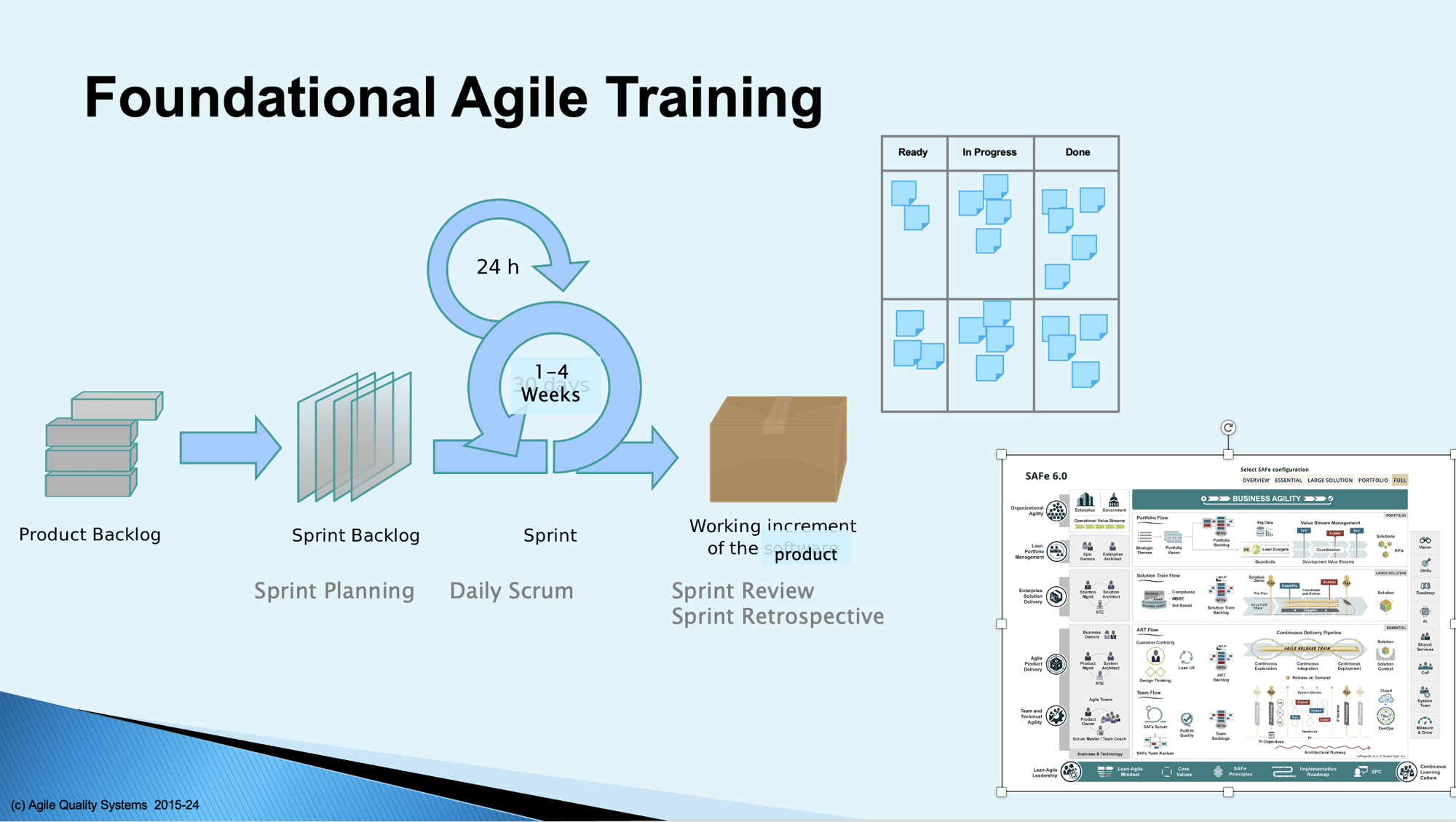
Customized training on the above and other topics can be provided, developed after an initial assessment of your organization's current state and desired improvement areas. I have delivered variations of this course more than 50 times, and they are never the same, with topics and depth guided by the needs of the audience.
For larger organizations building complex systems with more than one Agile team, the Scaled Agile Framework (SAFe®) augments the foundational Agile mechanisms of Scrum and Kanban to bring Agility to the enterprise.
As a SAFe® Program Consultant (SPC) since 2016, Kelly has delivered more than 80 SAFe® courses to more than 1600 participants, bringing both a solid academic understanding of SAFe® principles and practices, and a pragmatic approach to making SAFe® work in your context.
Customized workshops topic-specific sessions are available, including:
While training is important to establish foundational understanding of Agile principles and practices, the real learning happens by "doing" Agile, by "being" Agile.
Agile Quality Systems coaches teams and organizations in the mechanisms of Agile, to anchor the learning and establish good habits. This typically includes:

Agile Software Development is well established as an effective way to produce software quickly and effectively, but medical device software developers have concerns about the suitability of Agile. As a pioneer in the application of Agile to medical device software, I can address those concerns to help organizations find the advantages of Agile while maintaining high-quality software and compliant development processes.
As one of the lead authors for "AAMI TIR45 Guidance on the use of AGILE practices in the development of medical device software" and lead instructor for the associated AAMI Course (link to AAMI site), I provide industry-specific guidance on the safe and effective use of Agile.
Training options include:

For organizations new to the medical device world, or those who need a refresher, Agile Quality Systems provides foundational training and advanced coaching on the FDA Quality System Regulation and related standards.

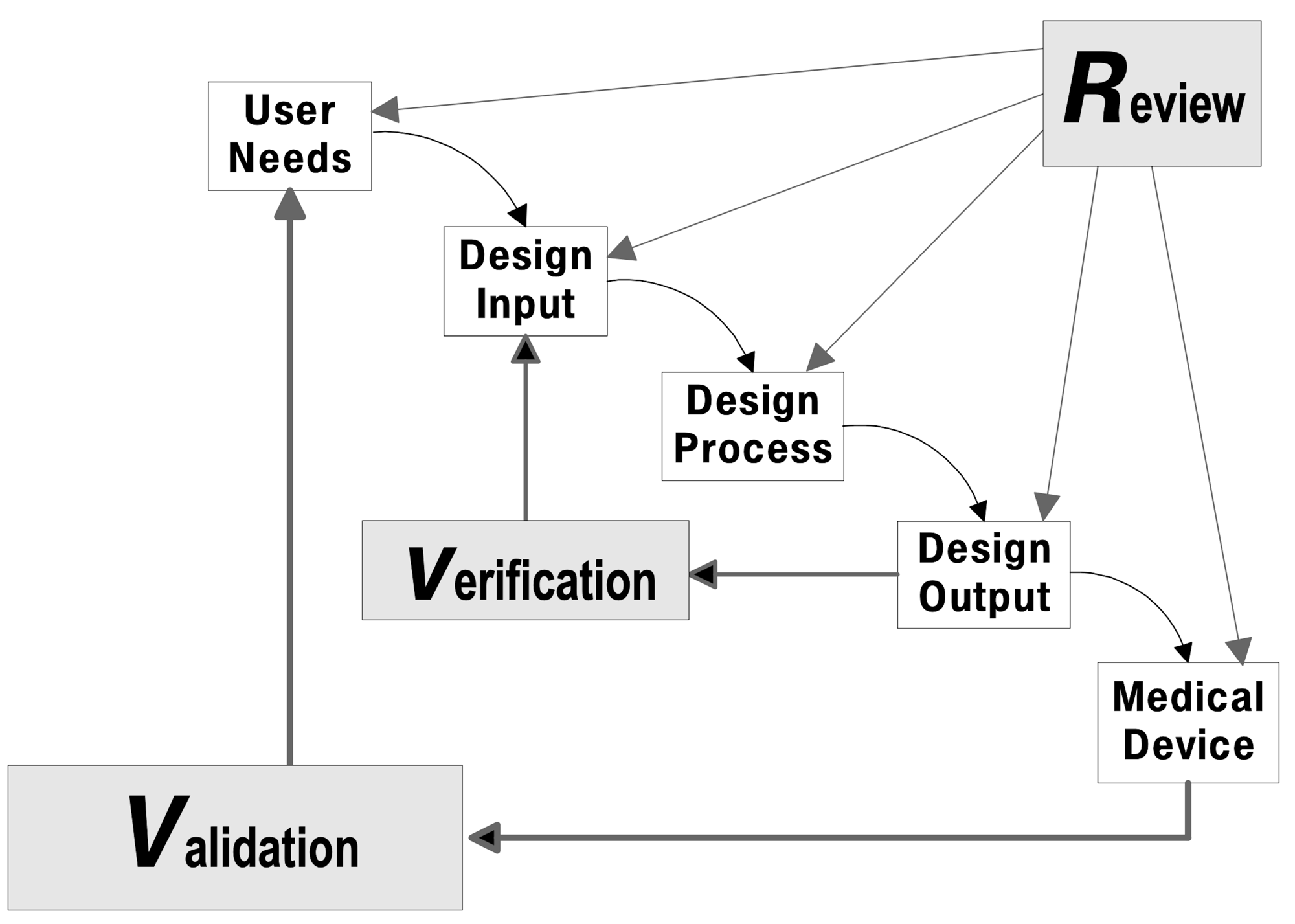
For medical device development organizations motivated to improve their quality management system (QMS), Agile Quality Systems can help. Whether the motivation comes from issues identified by regulators, or an internally identified need to improve, I work with the development team to identify opportunities and provide specific solutions to be implemented within the organization’s time, budget, and capacity constraints.
Focusing on Design Controls and related elements of the QMS, I provide a comprehensive and simple set of procedures and templates for software, hardware, and systems development, focusing on compliance to ISO 13485, FDA 21CFR820.30, and IEC 62304.
As an experienced creator and user of product development processes, I focus on practical procedures that fit with the organization's context and the product's risk level.

Audits and Assessments provide information to management about the organization's level of performance, and identifies risks related to regulatory certifications, regulatory submissions, or regulatory inspections. This information can be used to identify corrections and remediation activities that are needed before engaging with regulatory agencies. It can also be used as input to Quality System Improvement activities described above.
As an experienced and auditor (former American Society of Quality (ASQ) Certified Quality Auditor) specializing in ISO 13485, IEC 62304, ISO 14971, and FDA 21CFR820, I can lead or join a team to audit many elements of a Quality Management System, including Design Controls, CAPA, Risk Management, Complaint Handling, and others.
As an experienced product developer and auditee, I know how to avoid trivial observations and focus on providing audit results that help organizations improve their processes and their compliance to regulations.
©2026 Agile Quality Systems. All rights reserved. Privacy Policy